Sponsors of clinical trials employ individuals, known as site monitors, to visit clinics, hospitals, and other facilities and inspect the site and collect documentation for a given trial. Typically, a site monitor will make copies of paper documents during their visit, drive back to their office, and scan in the paper copies, send them by snail mail to a centralized scanning facility, or even fax them for inclusion in the eTMF. This is a time consuming and error prone process which we have greatly improved.
The Site Monitor Mobile Application allows the user to automate this process by providing an easy to use iOS app which can directly upload documents into the Documentum eTMF repository. Monitors can use their iOS device as a “mobile scanner” by taking photographs of the documents they need to collect, identifying what type of document it is, and have the document uploaded via their cellular or wifi connection.
My role in this project: Principal user experience designer, visual designer, project needs discovery and documentation, use-case development, user research, and usability studies.
Complex storyboards were used to informed the discovery process and stakeholder interviews to develop goals and structure of the information needs.
(created using Balsamiq)
High-definition interactive prototypes were used for engineering information and user acceptance research.
(created using Axure)
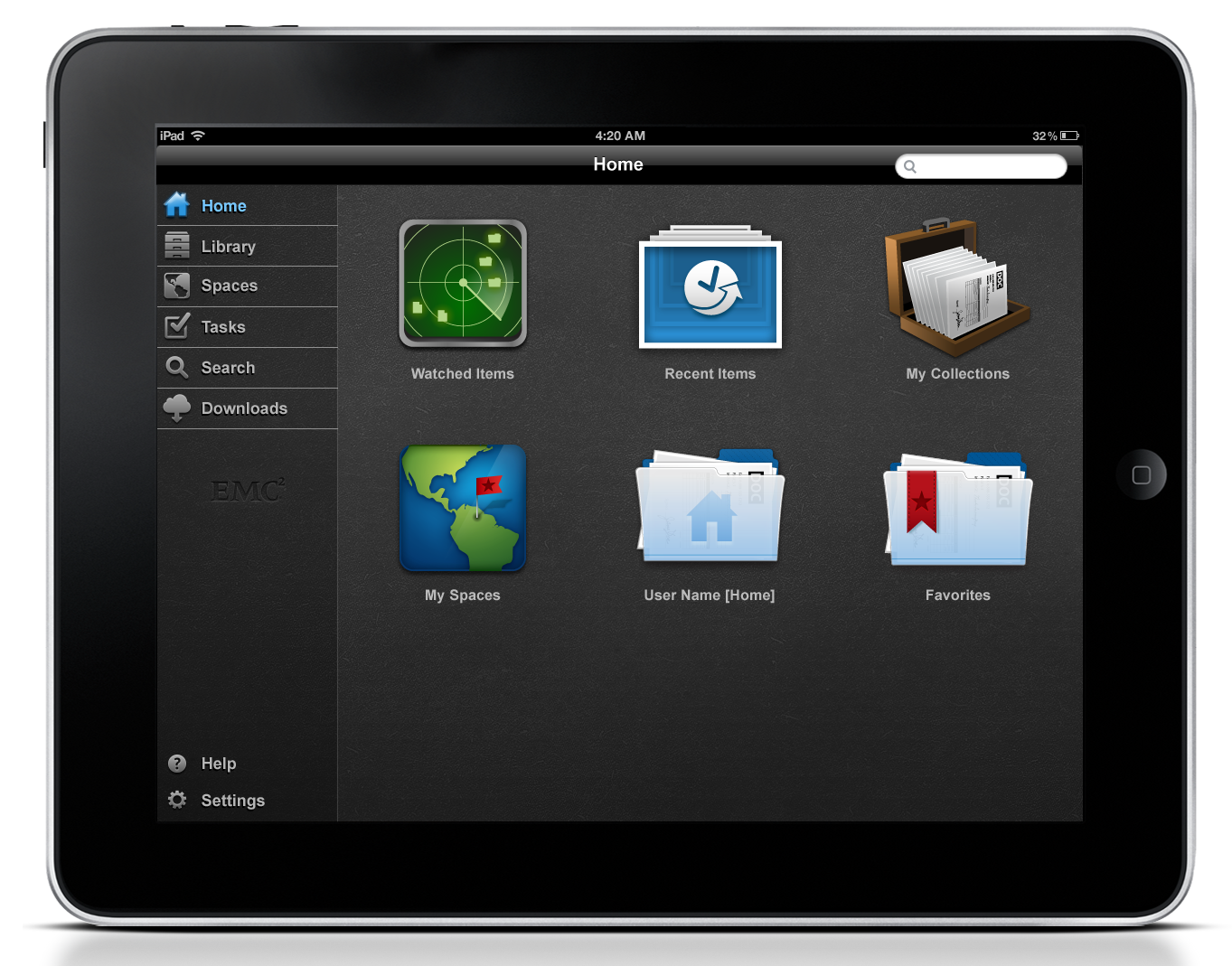
Home screen allows to select a clinical trial location.
A user may browse all documents by all locations.
Users may capture a document scan then edit and manage the document prior to sending to the repository.
change site location from any screen view.
Below are some of the UX architecture and style guide examples.
Application Branding Designs
Examples of the beta product development.